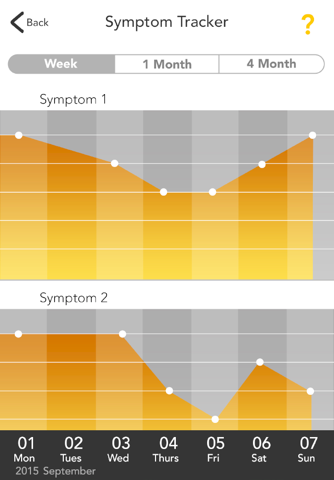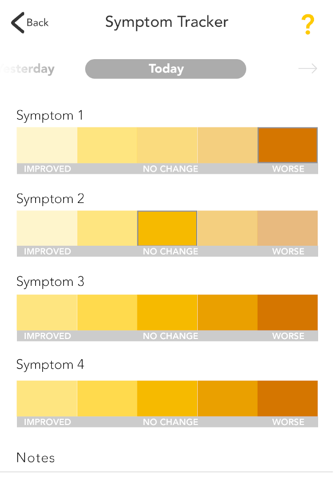
The GOtezla app offers helpful resources and support you may need during the first 30 days of treatment and beyond with Otezla® (apremilast). Plus, the tracking features make it easy for you to log your symptoms and share any changes with your doctor during office visits.
How GOtezla can help:
• Get personalized information that lets you know what to expect
• Track your progress with photographs, graphs, and notes to show your doctor during office visits
• Treatment reminders to help make sure you don’t miss a dose
• Access to nurses who’ll answer your questions 24 hours a day/7 days a week
GOtezla was developed with the help of people who are currently taking Otezla. When you’re starting treatment, GOtezla puts the tools you need at your fingertips.
Approved Uses
Otezla is a prescription medicine approved for the treatment of patients with moderate to severe plaque psoriasis for whom phototherapy or systemic therapy is appropriate.
Otezla is also approved for the treatment of adult patients with active psoriatic arthritis.
IMPORTANT SAFETY INFORMATION
You must not take Otezla if you are allergic to apremilast or to any of the ingredients in Otezla.
Otezla is associated with an increase in adverse reactions of depression. In clinical studies, some patients reported depression and suicidal behavior while taking Otezla. Some patients stopped taking Otezla due to depression. Before starting Otezla, tell your doctor if you have had feelings of depression, suicidal thoughts, or suicidal behavior. Be sure to tell your doctor if any of these symptoms or other mood changes develop or worsen during treatment with Otezla.
Some patients taking Otezla lost body weight. Your doctor should monitor your weight regularly. If unexplained or significant weight loss occurs, your doctor will decide if you should continue taking Otezla.
Some medicines may make Otezla less effective, and should not be taken with Otezla. Tell your doctor about all the medicines you take, including prescription and nonprescription medicines.
Side effects of Otezla in psoriasis clinical studies were diarrhea, nausea, upper respiratory tract infection, tension headache, and headache.
Side effects of Otezla in psoriatic arthritis clinical studies were diarrhea, nausea, and headache.
These are not all the possible side effects with Otezla. Ask your doctor about other potential side effects. Tell your doctor about any side effect that bothers you or does not go away.
Tell your doctor if you are pregnant, planning to become pregnant, or planning to breastfeed. Otezla has not been studied in pregnant women or in women who are breastfeeding.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-332-1088.
Please see Full Prescribing Information at www.otezla.com/otezla-prescribing-information.pdf.
As a reminder, only you and your doctor can determine what treatment is right for you. This app does not replace any medical advice provided by your healthcare provider. It is important that you discuss your treatment, treatment results, and all side effects with your healthcare provider. This app is not a medical device.
Data rates from your carrier may apply.
This application is brought to you by Celgene and is intended for use by plaque psoriasis and psoriatic arthritis patients, their caregivers, and their healthcare team. The design of this app is owned by Celgene Corporation and is subject to copyright protection. Celgene does not collect personal information from app users except as described in the GOtezla app Privacy Policy.
Otezla® is a registered trademark of Celgene Corporation.
© 2016 Celgene Corporation 10/16 USII-APR160006(3)